Resumen
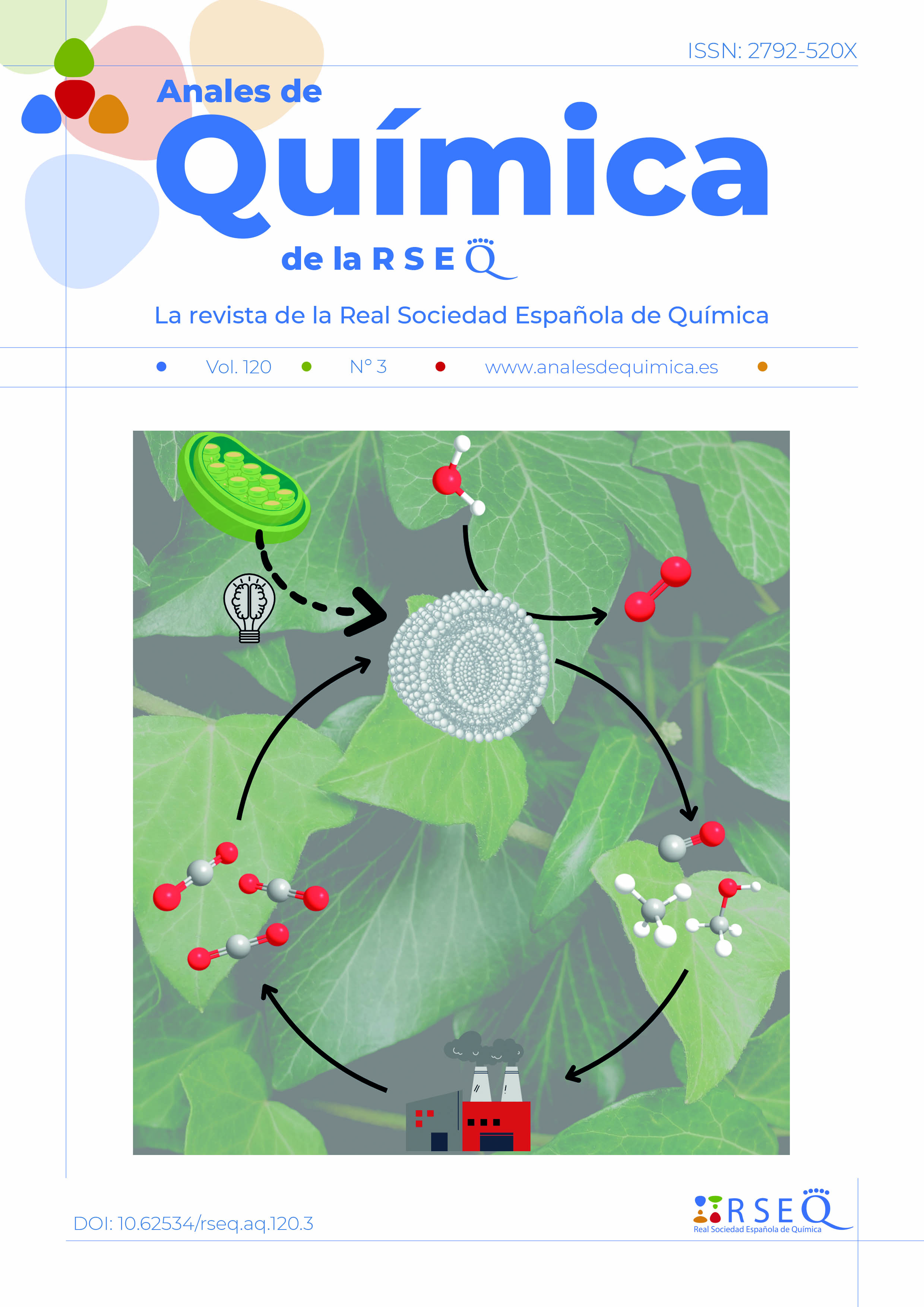
La fotosíntesis artificial tiene como objetivo imitar la fotosíntesis natural al almacenar energía solar en los enlaces químicos de combustibles y productos químicos, utilizando bloques de construcción simples y fácilmente disponibles como el agua y el dióxido de carbono. El desarrollo de sistemas fotocatalíticos eficientes y robustos para la fotosíntesis artificial requiere una comprensión exhaustiva de los mecanismos catalíticos subyacentes y de los factores que rigen la actividad y selectividad catalítica. Esta revisión enfatiza el creciente interés en el uso de vesículas artificiales bioinspiradas para compartimentar las transformaciones relacionadas con la fotosíntesis artificial. Aquí, resumimos los diferentes andamios utilizados para desarrollar vesículas artificiales bioinspiradas y exploramos ejemplos recientes en los que esos sistemas se han utilizado para estudiar procesos fotocatalíticos.
Referencias
Alavi, M.; Karimi, N.; Safaei, M. Application of Various Types of Liposomes in Drug Delivery Systems. Adv Pharm Bull 2017, 7, 3-9. https://doi.org/10.15171/apb.2017.002.
Altamura, E.; Milano, F.; Tangorra, R. R.; Trotta, M.; Omar, O. H.; Stano, P.; Mavelli, F. Highly oriented photosynthetic reaction centers generate a proton gradient in synthetic protocells. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 3837-3842. https://doi.org/10.1073/pnas.1617593114.
Antoku, D.; Satake, S.; Mae, T.; Sugikawa, K.; Funabashi, H.; Kuroda, A.; Ikeda, A. Improvement of Photodynamic Activity of Lipid–Membrane-Incorporated Fullerene Derivative by Combination with a Photo-Antenna Molecule. Chem. Eur. J. 2018, 24, 7335-7339. https://doi.org/10.1002/chem.201800674.
Askes, S. H. C.; Bahreman, A.; Bonnet, S. Activation of a Photodissociative Ruthenium Complex by Triplet–Triplet Annihilation Upconversion in Liposomes. Angew. Chem. Int. Ed. 2014, 53, 1029-1033. https://doi.org/10.1002/anie.201309389.
Askes, S. H. C.; Kloz, M.; Bruylants, G.; Kennis, J. T. M.; Bonnet, S. Triplet–triplet annihilation upconversion followed by FRET for the red light activation of a photodissociative ruthenium complex in liposomes. Phys. Chem. Chem. Phys. 2015, 17, 27380-27390. https://doi.org/10.1039/C5CP04352B.
Askes, S. H. C.; Meijer, M. S.; Bouwens, T.; Landman, I.; Bonnet, S. Red Light Activation of Ru(II) Polypyridyl Prodrugs via Triplet-Triplet Annihilation Upconversion: Feasibility in Air and through Meat. Molecules. 2016, 21, 1460. https://doi.org/10.3390/molecules21111460.
Beller, J.-N.; Beller, M. Spiers Memorial Lecture. Faraday Discuss. 2019, 215, 9-14. https://doi.org/10.1039/C9FD90025J.
Bhosale, S.; Sisson, A. L.; Talukdar, P.; Fürstenberg, A.; Banerji, N.; Vauthey, E.; Bollot, G.; Mareda, J.; Röger, C.; Würthner, F.; Sakai, N.; Matile, S. Photoproduction of Proton Gradients with π-Stacked Fluorophore Scaffolds in Lipid Bilayers. Science 2006, 313, 84-86. https://doi.org/10.1126/science.1126524.
Blankenship, R. E. Molecular mechanisms of photosynthesis; John Wiley & Sons, 2021.
Bonchio, M.; Gobbato, T.; Volpato, G. A.; Sartorel, A. Chem. Sci. 2023.
Calvin, M. Artificial photosynthesis. J. Membr. Sci. 1987, 33, 137-149. https://doi.org/10.1016/S0376-7388(00)80373-7.
Calvin, M. Simulating photosynthetic quantum conversion. Acc. Chem. Res. 1978, 11, 369-374. https://doi.org/10.1021/ar50130a001.
Casadevall, A. C; Codolà, Z.; Acuña-Parés, F.; Lloret-Fillol, J. An. Quim. 2016, 112, 133-141.
Casadevall, C. Heterogenization of Molecular Water Oxidation Catalysts in Electrodes for (Photo)Electrochemical Water Oxidation. Water 2022, 14, 371. https://doi.org/10.3390/w14030371.
Catania, R.; Machin, J.; Rappolt, M.; Muench, S. P.; Beales, P. A.; Jeuken, L. J. C. Detergent-Free Functionalization of Hybrid Vesicles with Membrane Proteins Using SMALPs. Macromolecules 2022, 55, 3415-3422. https://doi.org/10.1021/acs.macromol.2c00326.
Chang, T. M. S. 50th Anniversary of Artificial Cells: Their Role in Biotechnology, Nanomedicine, Regenerative Medicine, Blood Substitutes, Bioencapsulation, Cell/Stem Cell Therapy and Nanorobotics. Artif. Cells, Blood Substit. Biotechnol. 2007, 35, 545-554. https://doi.org/10.1080/10731190701730172.
Chang, T. M. S. ARTIFICIAL CELL evolves into nanomedicine, biotherapeutics, blood substitutes, drug delivery, enzyme/gene therapy, cancer therapy, cell/stem cell therapy, nanoparticles, liposomes, bioencapsulation, replicating synthetic cells, cell encapsulation/scaffold, biosorbent/immunosorbent haemoperfusion/plasmapheresis, regenerative medicine, encapsulated microbe, nanobiotechnology, nanotechnology. Artif. Cells Nanomed., Biotechnol. 2019, 47, 997-1013. https://doi.org/10.1080/21691401.2019.1577885.
Chen, B.; Chen, W.; Wang, M.; Peng, C. Unravelling the Multiple Synergies in MOF/CMP Supramolecular Heterojunction for Enhanced Artificial Photosynthesis. Adv. Mater. Interfaces 2023, 10, 2201971. https://doi.org/10.1002/admi.202201971.
Choi, H.-J.; Montemagno, C. D. Artificial Organelle: ATP Synthesis from Cellular Mimetic Polymersomes. Nano Lett. 2005, 5, 2538-2542. https://doi.org/10.1021/nl051896e.
Cox, N.; Pantazis, D. A.; Lubitz, W. Current Understanding of the Mechanism of Water Oxidation in Photosystem II and Its Relation to XFEL Data. Annu. Rev. Biochem. 2020, 89, 795-820. https://doi.org/10.1146/annurev-biochem-011520-104801.
Discher, B. M.; Won, Y. Y.; Ege, D. S.; Lee, J. C. M.; Bates, F. S.; Discher, D. E.; Hammer, D. A. Polymersomes: Tough Vesicles Made from Diblock Copolymers. Science 1999, 284, 1143-1146. https://doi.org/10.1126/science.284.5417.1143.
Domingues, J. M.; Miranda, C. S.; Homem, N. C.; Felgueiras, H. P.; Antunes, J. C. Nanoparticle Synthesis and Their Integration into Polymer-Based Fibers for Biomedical Applications. Biomedicines 2023, 11, 1862. https://doi.org/10.3390/biomedicines11071862.
Dubed Bandomo, G. C.; Mondal, S. S.; Franco, F.; Bucci, A.; Martin-Diaconescu, V.; Ortuño, M. A. ; Langevelde, P. H. van; Shafir, A.; López, N.; Lloret-Fillol, J. Mechanically Constrained Catalytic Mn(CO)3Br Single Sites in a Two-Dimensional Covalent Organic Framework for CO2 Electroreduction in H2O. ACS Cat. 2021, 11, 7210-7222. https://doi.org/10.1021/acscatal.1c00314.
Egli, S.; Nussbaumer, M. G.; Balasubramanian, V.; Chami, M.; Bruns, N.; Palivan, C.; Meier, W. Biocompatible Functionalization of Polymersome Surfaces: A New Approach to Surface Immobilization and Cell Targeting Using Polymersomes. J. Am. Chem. Soc. 2011, 133, 4476-4483. https://doi.org/10.1021/ja110275f.
Elani, Y.; Law, R. V.; Ces, O. Vesicle-based artificial cells as chemical microreactors with spatially segregated reaction pathways. Nat. Comm. 2014, 5, 5305. https://doi.org/10.1038/ncomms6305.
Emir Diltemiz, S.; Tavafoghi, M.; de Barros, N. R.; Kanada, M.; Heinämäki, J.; Contag, C.; Seidlits, S. K.; Ashammakhi, N. Use of artificial cells as drug carriers. Mater. Chem. Front. 2021, 5, 6672-6692. https://doi.org/10.1039/D1QM00717C.
Erb, T. J.; Zarzycki, J. A short history of RubisCO: the rise and fall (?) of Nature's predominant CO2 fixing enzyme. Curr. Opin. Biotechnol. 2018, 49, 100-107. https://doi.org/10.1016/j.copbio.2017.07.017.
Fang, X.; Kalathil, S.; Reisner, E. Semi-biological approaches to solar-to-chemical conversion. Chem. Soc. Rev. 2020, 49, 4926-4952. https://doi.org/10.1039/C9CS00496C.
Ford, W. E.; Otvos, J. W.; Calvin, M. Photosensitised electron transport across phospholipid vesicle walls. Nature 1978, 274, 507-508. https://doi.org/10.1038/274507a0.
Ford, W. E.; Otvos, J. W.; Calvin, M. Photosensitized electron transport across lipid vesicle walls: quantum yield dependence on sensitizer concentration. Proc. Natl. Acad. Sci. 1979, 76, 3590. https://doi.org/10.1073/pnas.76.8.3590.
Fujita, S.; Motoda, Y.; Kigawa, T.; Tsuchiya, K.; Numata, K. Peptide-Based Polyion Complex Vesicles That Deliver Enzymes into Intact Plants To Provide Antibiotic Resistance without Genetic Modification. Biomacromolecules 2021, 22, 1080-1090. https://doi.org/10.1021/acs.biomac.0c01380.
Gaitzsch, J.; Huang, X.; Voit, B. Engineering Functional Polymer Capsules toward Smart Nanoreactors. Chem. Rev. 2016, 116, 1053-1093. https://doi.org/10.1021/acs.chemrev.5b00241.
Garni, M.; Thamboo, S.; Schoenenberger, C. A.; Palivan, C. G. Biopores/membrane proteins in synthetic polymer membranes. Biochim Biophys Acta Biomembr 2017, 1859, 619-638. https://doi.org/10.1016/j.bbamem.2016.10.015.
Gaur, D.; Dubey, N. C.; Tripathi, B. P. Biocatalytic self-assembled synthetic vesicles and coacervates: From single compartment to artificial cells. Adv. Colloid Interface Sci. 2022, 299, 102566. https://doi.org/10.1016/j.cis.2021.102566.
Ghorbanizamani, F.; Moulahoum, H.; Zihnioglu, F.; Timur, S. Nanohybrid carriers: the yin–yang equilibrium between natural and synthetic in biomedicine. Biomater. Sci. 2020, 8, 3237-3247. https://doi.org/10.1039/D0BM00401D.
Gobbato, T.; Rigodanza, F.; Benazzi, E.; Costa, P.; Garrido, M.; Sartorel, A.; Prato, M.; Bonchio, M. Enhancing Oxygenic Photosynthesis by Cross-Linked Perylenebisimide “Quantasomes”. J. Am. Chem. Soc. 2022, 144, 14021-14025. https://doi.org/10.1021/jacs.2c05857
Goto, A.; Anraku, Y.; Fukushima, S.; Kishimura, A. Increased Enzyme Loading in PICsomes via Controlling Membrane Permeability Improves Enzyme Prodrug Cancer Therapy Outcome. Polymers 2023, 15, 1368. https://doi.org/10.3390/polym15061368.
Grimaldi, J. J.; Boileau, S.; Lehn, J.-M. Light-driven, carrier-mediated electron transfer across artificial membranes. Nature 1977, 265, 229-230. https://doi.org/10.1038/265229a0.
Gumz, H.; Lai, T. H.; Voit, B.; Appelhans, D. Fine-tuning the pH response of polymersomes for mimicking and controlling the cell membrane functionality. Polym. Chem. 2017, 8, 2904- 2908. https://doi.org/10.1039/C7PY00089H.
Gust, D.; Moore, T. A. Mimicking Photosynthesis. Science 1989, 244, 35-41. https://doi.org/10.1126/science.244.4900.35.
Gutiérrez, L.; Martin-Diaconescu, V.; Casadevall, C.; Oropeza, F.; Peña O'Shea, V. A. de la; Meng, J.J.; Ortuño, M. A.; Lloret- Fillol, J. Low Oxidation State Cobalt Center Stabilized by a Covalent Organic Framework to Promote Hydroboration of Olefins. ACS Cat. 2023, 13, 3044-3054. https://doi.org/10.1021/acscatal.2c05442.
Ham, R.; Nielsen, C. J.; Pullen, S.; Reek, J. N. H. Supramolecular Coordination Cages for Artificial Photosynthesis and Synthetic Photocatalysis. Chem. Rev. 2023, 123, 5225-5261. https://doi.org/10.1021/acs.chemrev.2c00759.
Han, W.-K.; Liu, Y.; Yan, X.; Jiang, Y.; Zhang, J.; Gu, Z.-G. Integrating Light-Harvesting Ruthenium(II)-based Units into Three-Dimensional Metal Covalent Organic Frameworks for Photocatalytic Hydrogen Evolution. Angew. Chem. Int. Ed. 2022, 61, e202208791. https://doi.org/10.1002/anie.202208791
Hansen, M.; Li, F.; Sun, L.; König, B. Photocatalytic water oxidation at soft interfaces. Chem. Sci. 2014, 5, 2683-2687. https://doi.org/10.1039/C4SC01018C.
Hansen, M.; Troppmann, S.; König, B. Artificial Photosynthesis at Dynamic Self-Assembled Interfaces in Water. Chem. Eur. J. 2016, 22, 58-72. https://doi.org/10.1002/chem.201503712.
Hatae, T.; Koshiyama, T.; Ohba, M. Domain Size Dependent Fluorescence Resonance Energy Transfer in Lipid Domain Incorporated Fluorophores. Chem. Lett. 2017, 46, 756-759. https://doi.org/10.1246/cl.170104.
He, T.; Zhao, Z.; Liu, R.; Liu, X.; Ni, B.; Wei, Y.; Wu, Y.; Yuan, W.; Peng, H.; Jiang, Z.; Zhao, Y. Porphyrin-Based Covalent Organic Frameworks Anchoring Au Single Atoms for Photocatalytic Nitrogen Fixation. J. Am. Chem. Soc. 2023, 145, 6057-6066. https://doi.org/10.1021/jacs.2c10233
Heuberger, L.; Korpidou, M.; Eggenberger, O. M.; Kyropoulou, M.; Palivan, C. Current Perspectives on Synthetic Compartments for Biomedical Applications. G. Int. J. Mol. Sci. 2022, 23, 5718. https://doi.org/10.3390/ijms23105718.
Higashida, Y.; Takizawa, S.-y.; Yoshida, M.; Kato, M.; Kobayashi, A. Hydrogen Production from Hydrophobic Ruthenium Dye-Sensitized TiO2 Photocatalyst Assisted by Vesicle Formation. ACS Appl. Mater. Interfaces 2023, 15, 27277-27284. https://doi.org/10.1021/acsami.3c02340.
Hori, M.; Cabral, H.; Toh, K.; Kishimura, A.; Kataoka, K. Robust Polyion Complex Vesicles (PICsomes) under Physiological Conditions Reinforced by Multiple Hydrogen Bond Formation Derived by Guanidinium Groups. Biomacromolecules 2018, 19, 4113-4121. https://doi.org/10.1021/acs.biomac.8b01097.
Hu, H.; Wang, Z.; Cao, L.; Zeng, L.; Zhang, C.; Lin, W.; Wang, C. Metal–organic frameworks embedded in a liposome facilitate overall photocatalytic water splitting. Nat. Chem, 2021, 13, 358-366. https://doi.org/10.1038/s41557-020-00635-5.
Hu, S.; Yan, J.; Yang, G.; Ma, C.; Yin, J. Self-Assembled Polymeric Materials: Design, Morphology, and Functional-Oriented Applications. Macromol. Rapid Commun. 2022, 43, 2100791. https://doi.org/10.1002/marc.202100791.
Huang, X.; Li, M.; Green, D. C.; Williams, D. S.; Patil, A. J.; Mann, S. Interfacial assembly of protein–polymer nano-conjugates into stimulus-responsive biomimetic protocells. Nat. Comm. 2013, 4, 2239. https://doi.org/10.1038/ncomms3239.
Hvasanov, D.; Peterson, J. R.; Thordarson, P. Self-assembled light-driven photosynthetic-respiratory electron transport chain hybrid proton pump. Chem. Sci. 2013, 4, 3833-3838. https://doi.org/10.1039/c3sc51780b.
Ikuta, N.; Takizawa, S.-y.; Murata, S. Photochemical reduction of CO2 with ascorbate in aqueous solution using vesicles acting as photocatalysts. Photochem. Photobiol. Sci. 2014, 13, 691-702. https://doi.org/10.1039/c3pp50429h.
Infelta, P. P.; Graetzel, M.; Fendler, J. H. Aspects of artificial photosynthesis. Photosensitized electron transfer and charge separation in cationic surfactant vesicles. J. Am. Chem. Soc. 1980, 102, 1479-1483. https://doi.org/10.1021/ja00525a001.
Jacobi, R.; Hernández-Castillo, D.; Sinambela, N.; Bösking, J.; Pannwitz, A.; González, L. Computation of Förster Resonance Energy Transfer in Lipid Bilayer Membranes. J. Phys. Chem. A 2022, 126, 8070-8081. https://doi.org/10.1021/acs.jpca.2c04524.
Jeong, S.; Nguyen, H. T.; Kim, C. H.; Ly, M. N.; Shin, K. Toward Artificial Cells: Novel Advances in Energy Conversion and Cellular Motility. Adv. Funct. Mater. 2020, 30, 1907182. https://doi.org/10.1002/adfm.201907182
Jia, H.; Schwille, P. Bottom-up synthetic biology: reconstitution in space and time. Curr. Opin. Biotechnol. 2019, 60, 179-187. https://doi.org/10.1016/j.copbio.2019.05.008.
Keijer, T.; Bouwens, T.; Hessels, J.; Reek, J N. H. Supramolecular strategies in artificial photosynthesis. Chem. Sci. 2021, 12, 50-70. https://doi.org/10.1039/D0SC03715J.
Kishimura, A. Development of polyion complex vesicles (PICsomes) from block copolymers for biomedical applications. Polym. J. 2013, 45, 892-897. https://doi.org/10.1038/pj.2013.33.
Klein, D. M.; Passerini, L.; Huber, M.; Bonnet, S. A Stable Alkylated Cobalt Catalyst for Photocatalytic H2 Generation in Liposomes. ChemCatChem 2022, 14, e202200484. https://doi.org/10.1002/cctc.202200484.
Klein, D. M.; Rodríguez-Jiménez, S.; Hoefnagel, M. E.; Pannwitz, A.; Prabhakaran, A.; Siegler, M. A.; Keyes, T. E.; Reisner, E.; Brouwer, A. M.; Bonnet, S. Shorter Alkyl Chains Enhance Molecular Diffusion and Electron Transfer Kinetics between Photosensitisers and Catalysts in CO2-Reducing Photocatalytic Liposomes. Chem. Eur. J. 2021, 27, 17203-17212. https://doi.org/10.1002/chem.202102989.
Kleineberg, C.; Wölfer, C.; Abbasnia, A.; Pischel, D.; Bednarz, C.; Ivanov, I.; Heitkamp, T.; Börsch, M.; Sundmacher, K.; Vidaković-Koch, T. Light-Driven ATP Regeneration in Diblock/Grafted Hybrid Vesicles. ChemBioChem 2020, 21, 2149-2160. https://doi.org/10.1002/cbic.201900774.
Klermund, L.; Castiglione, K. Polymersomes as nanoreactors for preparative biocatalytic applications: current challenges and future perspectives. Bioproc. Biosyst. Eng. 2018, 41, 1233-1246. https://doi.org/10.1007/s00449-018-1953-9.
Klermund, L.; Poschenrieder, S. T.; Castiglione, K. Biocatalysis in Polymersomes: Improving Multienzyme Cascades with Incompatible Reaction Steps by Compartmentalization. ACS Cat. 2017, 7, 3900-3904. https://doi.org/10.1021/acscatal.7b00776.
Kobayashi, A.; Takizawa, S.-y.; Hirahara, M. Photofunctional molecular assembly for artificial photosynthesis: Beyond a simple dye sensitization strategy. Coord. Chem. Rev. 2022, 467, 214624. https://doi.org/10.1016/j.ccr.2022.214624.
Kuang, L.; Olson, T. L.; Lin, S.; Flores, M.; Jiang, Y.; Zheng, W.; Williams, J. C.; Allen, J. P.; Liang, H. Interface for Light-Driven Electron Transfer by Photosynthetic Complexes Across Block Copolymer Membranes. J. Phys. Chem. Let. 2014, 5, 787-791. https://doi.org/10.1021/jz402766y.
Kurihara, K.; Fendler, J. H. Electron-transfer catalysis by surfactant vesicle stabilized colloidal platinum. J. Am. Chem. Soc. 1983, 105, 6152-6153. https://doi.org/10.1021/ja00357a032.
Kwolek, U.; Nakai, K.; Pluta, A.; Zatorska, M.; Wnuk, D.; Lasota, S.; Bednar, J.; Michalik, M.; Yusa, S. I.; Kepczynski, M. Polyion complex vesicles (PICsomes) from strong copolyelectrolytes. Stability and in vitro studies. Colloids Surf B Biointerfaces 2017, 158, 658-666. https://doi.org/10.1016/j.colsurfb.2017.07.042.
Le Meins, J.-F.; Sandre, O.; Lecommandoux, S. Recent trends in the tuning of polymersomes’ membrane properties. Eur. Phys. J. E 2011, 34, 1-17. https://doi.org/10.1140/epje/i2011-11014-y.
Lee, K. Y.; Park, S.-J.; Lee, K. A.; Kim, S.-H.; Kim, H.; Meroz, Y.; Mahadevan, L.; Jung, K.-H.; Ahn, T. K.; Parker, K. K.; Shin, K. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system. Nat. Biotechnol. 2018, 36, 530-535. https://doi.org/10.1038/nbt.4140.
Lee, K. Y.; Park, S.-J.; Lee, K. A.; Kim, S.-H.; Kim, H.; Meroz, Y.; Mahadevan, L.; Jung, K.-H.; Ahn, T. K.; Parker, K. K.; Shin, K. Nat. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system Biotechnol. 2018, 36, 530-535. https://doi.org/10.1038/nbt.4140.
Lewis, N. S. Developing a scalable artificial photosynthesis technology through nanomaterials by design. Nat. Nanotechnol. 2016, 11, 1010-1019. https://doi.org/10.1038/nnano.2016.194.
Li, H.; Liu, Y.; Huang, T.; Qi, M.; Ni, Y.; Wang, J.; Zheng, Y.; Zhou, Y.; Yan, D. Construction of Light-Harvesting Polymeric Vesicles in Aqueous Solution with Spatially Separated Donors and Acceptors. Macromol. Rapid Commun. 2017, 38, 1600818. https://doi.org/10.1002/marc.201600818.
Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian Journal of Pharmaceutical Sciences 2015, 10, 81-98. https://doi.org/10.1016/j.ajps.2014.09.004.
Li, M.; Green, D. C.; Anderson, J. L. R.; Binks, B. P.; Mann, S. In vitro gene expression and enzyme catalysis in bio-inorganic protocells. Chem. Sci. 2011, 2, 1739-1745. https://doi.org/10.1039/c1sc00183c.
Li, Y.; Liu, L.; Zhao, H. Enzyme-catalyzed cascade reactions on multienzyme proteinosomes. Journal of Colloid and Interface Science 2022, 608, 2593-2601. https://doi.org/10.1016/j.jcis.2021.10.185.
Life after the synthetic cell. Nature 2010, 465, 422-424. https://doi.org/10.1038/465422a.
Limburg, B.; Bouwman, E.; Bonnet, S. Catalytic photoinduced electron transport across a lipid bilayer mediated by a membrane-soluble electron relay. Chem. Commun. 2015, 51, 17128-17131. https://doi.org/10.1039/C5CC07745A.
Limburg, B.; Wermink, J.; van Nielen, S. S.; Kortlever, R.; Koper, M. T. M.; Bouwman, E.; Bonnet, S. Kinetics of Photocatalytic Water Oxidation at Liposomes: Membrane Anchoring Stabilizes the Photosensitizer. ACS Cat. 2016, 6, 5968-5977. https://doi.org/10.1021/acscatal.6b00151.
Lopez, A.; Fiore, M. Investigating Prebiotic Protocells for a Comprehensive Understanding of the Origins of Life: A Prebiotic Systems Chemistry Perspective. Life 2019, 9, 49. https://doi.org/10.3390/life9020049.
LoPresti, C.; Lomas, H.; Massignani, M.; Smart, T.; Battaglia, G. Polymersomes: nature inspired nanometer sized compartments. J. Mater. Chem. 2009, 19, 3576-3590. https://doi.org/10.1039/b818869f.
Lorenceau, E.; Utada, A. S.; Link, D. R.; Cristobal, G.; Joanicot, M.; Weitz, D. A. Generation of Polymerosomes from Double-Emulsions. Langmuir 2005, 21, 9183-9186. https://doi.org/10.1021/la050797d.
Lubitz, W.; Ogata, H.; Rüdiger, O.; Reijerse, E. Hydrogenases. Chem. Rev. 2014, 114, 4081-4148. https://doi.org/10.1021/cr4005814.
Lubitz, W.; Pantazis, D. A.; Cox, N. Water oxidation in oxygenic photosynthesis studied by magnetic resonance techniques. FEBS Lett. 2023, 597, 6-29. https://doi.org/10.1002/1873-3468.14543
Mansy, S. S.; Szostak, J. W. Reconstructing the Emergence of Cellular Life through the Synthesis of Model Protocells. Cold Spring Harb. Symp. Quant. Biol. 2009, 74, 47-54. https://doi.org/10.1101/sqb.2009.74.014
Matos, M. B. C. de; Miranda, B. S.; Rizky Nuari, Y.; Storm, G.; Leneweit, G.; Schiffelers, R. M.; Kok, R. J. Liposomes with asymmetric bilayers produced from inverse emulsions for nucleic acid delivery. J. Drug Target 2019, 27, 681-689. https://doi.org/10.1080/1061186X.2019.1579819.
Meier, W.; Nardin, C.; Winterhalter, M. Reconstitution of Channel Proteins in (Polymerized) ABA Triblock Copolymer Membranes. Angew. Chem. Int. Ed. 2000, 39, 4599-4602. https://doi.org/10.1002/1521-3773(20001215)39:24<4599::AID-ANIE4599>3.0.CO;2-Y.
Meng, G.; Zhen, L.; Sun, S.; Hai, J.; Zhang, Z.; Sun, D.; Liu, Q.; Wang, B. Confining perovskite quantum dots in the pores of a covalent-organic framework: quantum confinement- and passivation-enhanced light-harvesting and photocatalysis. J. Mater. Chem. A 2021, 9, 24365-24373. https://doi.org/10.1039/D1TA07733C.
Miller, T. E.; Beneyton, T.; Schwander, T.; Diehl, C.; Girault, M.; McLean, R.; Chotel, T.; Claus, P.; Cortina, N. S.; Baret, J.-C.; Erb, T. J. Light-powered CO2 fixation in a chloroplast mimic with natural and synthetic parts. Science 2020, 368, 649-654. https://doi.org/10.1126/science.aaz6802.
Mulla, Y.; Aufderhorst-Roberts, A.; Koenderink, G. H. Shaping up synthetic cells. Phys. Biol. 2018, 15, 041001. https://doi.org/10.1088/1478-3975/aab923.
Namiki, Y.; Fuchigami, T.; Tada, N.; Kawamura, R.; Matsunuma, S.; Kitamoto, Y.; Nakagawa, M. Nanomedicine for Cancer: Lipid-Based Nanostructures for Drug Delivery and Monitoring. Acc. Chem. Res. 2011, 44, 1080-1093. https://doi.org/10.1021/ar200011r.
Nardin, C.; Thoeni, S.; Widmer, J.; Winterhalter, M.; Meier, W. Nanoreactors based on (polymerized) ABA-triblock copolymer vesicles. Chem. Commun. 2000, 1433-1434. https://doi.org/10.1039/b004280n.
Nau, R. E. P.; Bösking, J.; Pannwitz, A. Compartmentalization Accelerates Photosensitized NADH to NAD+ Conversion. ChemPhotoChem 2022, 6, e202200158. https://doi.org/10.1002/cptc.202200158.
Navarro, M. Á.; Cosano, D.; Bhunia, A.; Simonelli, L.; Martin-Diaconescu, V.; Romero-Salguero, F. J.; Esquivel, D. Sustain. Cobaloxime tethered pyridine-functionalized ethylene-bridged periodic mesoporous organosilica as an efficient HER catalyst. Energy Fuels 2022, 6, 398-407. https://doi.org/10.1039/D1SE01437D.
Nelson, N.; Ben-Shem, A. The complex architecture of oxygenic photosynthesis. Nat. Rev. Mol. Cell Biol. 2004, 5, 971-982. https://doi.org/10.1038/nrm1525.
Osaki, T.; Takeuchi, S. Artificial Cell Membrane Systems for Biosensing Applications. Anal. Chem. 2017, 89, 216-231. https://doi.org/10.1021/acs.analchem.6b04744.
Palivan, C. G.; Goers, R.; Najer, A.; Zhang, X.; Car, A.; Meier, W. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 2016, 45, 377-411. https://doi.org/10.1039/C5CS00569H
Pannwitz, A.; Klein, D. M.; Rodríguez-Jiménez, S.; Casadevall, C.; Song, H.; Reisner, E.; Hammarström, L.; Bonnet, S. Roadmap towards solar fuel synthesis at the water interface of liposome membranes. Chem. Soc. Rev. 2021, 50, 4833-4855. https://doi.org/10.1039/D0CS00737D.
Pannwitz, A.; Saaring, H.; Beztsinna, N.; Li, X.; Siegler, M. A.; Bonnet, S. Mimicking Photosystem I with a Transmembrane Light Harvester and Energy Transfer-Induced Photoreduction in Phospholipid Bilayers. Chem. Eur. J. 2021, 27, 3013-3018. https://doi.org/10.1002/chem.202003391.
Parambil, S. R. V.; Karmakar, S.; Rahimi, F. A.; Maji, T. K. Confining Molecular Photosensitizer and Catalyst in MOF toward Artificial Photosynthesis: Validating Electron Transfer by In Situ DRIFT Study. ACS Appl. Mater. Interfaces 2023, 15, 27821-27831. https://doi.org/10.1021/acsami.3c01153.
Park, R. B.; Biggins, J. Quantasome: Size and Composition. Science 1964, 144, 1009-1011. https://doi.org/10.1126/science.144.3621.1009.
Pata, V.; Dan, N. The Effect of Chain Length on Protein Solubilization in Polymer-Based Vesicles (Polymersomes). Biophys. J. 2003, 85, 2111-2118. https://doi.org/10.1016/S0006-3495(03)74639-6.
Piper, S. E. H.; Casadevall, C.; Reisner, E.; Clarke, T. A.; Jeuken, L. J. C.; Gates, A. J.; Butt, J. N. Photocatalytic Removal of the Greenhouse Gas Nitrous Oxide by Liposomal Microreactors. Angew. Chem. Int. Ed. 2022, 61, e202210572. https://doi.org/10.1002/anie.202210572.
Piper, S. E. H.; Edwards, M. J.; van Wonderen, J. H.; Casadevall, C.; Martel, A.; Jeuken, L. J. C.; Reisner, E.; Clarke, T. A.; Butt, J. N. Bespoke Biomolecular Wires for Transmembrane Electron Transfer: Spontaneous Assembly of a Functionalized Multiheme Electron Conduit. Front. Microbiol. 2021, 12. https://doi.org/10.3389/fmicb.2021.714508.
Ran, L.; Li, Z.; Ran, B.; Cao, J.; Zhao, Y. ; Shao, T.; Song, Y. ; Leung, M. K. H.; Sun, L.; Hou, J. Engineering Single-Atom Active Sites on Covalent Organic Frameworks for Boosting CO2 Photoreduction. J. Am. Chem. Soc. 2022, 144, 17097-17109. https://doi.org/10.1021/jacs.2c06920.
Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F. R.; Landfester, K. Liposomes and polymersomes: a comparative review towards cell mimicking. Chem. Soc. Rev. 2018, 47, 8572-8610. https://doi.org/10.1039/C8CS00162F.
Rodríguez-García, R.; Mell, M.; López-Montero, I.; Netzel, J.; Hellweg, T.; Monroy, F. Polymersomes: smart vesicles of tunable rigidity and permeability. Soft Matter 2011, 7, 1532-1542. https://doi.org/10.1039/c0sm00823k.
Rodríguez-Jiménez, S.; Song, H.; Lam, E.; Wright, D.; Pannwitz, A.; Bonke, S. A.; Baumberg, J. J.; Bonnet, S.; Hammarström, L.; Reisner, E. Self-Assembled Liposomes Enhance Electron Transfer for Efficient Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2022, 144, 9399-9412. https://doi.org/10.1021/jacs.2c01725.
Salehi-Reyhani, A.; Ces, O.; Elani, Y. Artificial cell mimics as simplified models for the study of cell biology. Exp Biol Med (Maywood) 2017, 242, 1309-1317. https://doi.org/10.1177/1535370217711441.
Sato, Y.; Takizawa, S.-y.; Murata, S. Photochemical water oxidation system using ruthenium catalysts embedded into vesicle membranes. J. Photoch. Photobio. A. 2016, 321, 151-160. https://doi.org/10.1016/j.jphotochem.2016.02.003.
Schlossarek, T.; Stepanenko, V.; Beuerle, F.; Würthner, F. Self-assembled Ru(bda) Coordination Oligomers as Efficient Catalysts for Visible Light-Driven Water Oxidation in Pure Water. Angew. Chem. Int. Ed. 2022, 61, e202211445. https://doi.org/10.1002/anie.202211445.
Schwander, T.; Schada von Borzyskowski, L.; Burgener, S.; Cortina, N. S.; Erb, T. A synthetic pathway for the fixation of carbon dioxide in vitro. J. Science 2016, 354, 900-904. https://doi.org/10.1126/science.aah5237.
Schwarzer, T. S.; Klermund, L.; Wang, G.; Castiglione, K. Membrane functionalization of polymersomes: alleviating mass transport limitations by integrating multiple selective membrane transporters for the diffusion of chemically diverse molecules. Nanotechnology 2018, 29, 44LT01. https://doi.org/10.1088/1361-6528/aadb7e.
Shafaat, H. S.; Yang, J. Y. Uniting biological and chemical strategies for selective CO2 reduction. Nat. Catal. 2021, 4, 928-933. https://doi.org/10.1038/s41929-021-00683-1.
Sharma, N.; Jose, D. A.; Jain, N.; Parmar, S.; Srivastav, A.; Chawla, J.; Naziruddin, A. R.; Mariappan, C. R. Regulation of Nitric Oxide (NO) Release by Membrane Fluidity in Ruthenium Nitrosyl Complex-Embedded Phospholipid Vesicles. Langmuir 2022, 38, 13602-13612. https://doi.org/10.1021/acs.langmuir.2c02457.
Shin, K. Artificial cells containing sustainable energy conversion engines. Emerg. Top. Life. Sci. 2019, 3, 573-578. https://doi.org/10.1042/ETLS20190103.
Sinambela, N.; Bösking, J.; Abbas, A. Pannwitz, A. Recent Advances in Light Energy Conversion with Biomimetic Vesicle Membranes. ChemBioChem 2021, 22, 3140-3147. https://doi.org/10.1002/cbic.202100220.
Sinambela, N.; Jacobi, R.; Hernández-Castillo, D.; Hofmeister, E.; Hagmeyer, N.; Dietzek-Ivanšić, B.; González, L.; Pannwitz, A. Alignment and photooxidation dynamics of a perylene diimide chromophore in lipid bilayers. Mol. Syst. Des. Eng. 2023, 8, 842-852. https://doi.org/10.1039/D2ME00243D.
Song, H.; Amati, A.; Pannwitz, A.; Bonnet, S.; Hammarström, L. Mechanistic Insights into the Charge Transfer Dynamics of Photocatalytic Water Oxidation at the Lipid Bilayer–Water Interface. J. Am. Chem. Soc. 2022, 144, 19353-19364. https://doi.org/10.1021/jacs.2c06842.
Steinberg-Yfrach, G.; Liddell, P. A.; Hung, S.-C.; Moore, A. L.; Gust, D.; Moore, T. A. Conversion of light energy to proton potential in liposomes by artificial photosynthetic reaction centres. Nature 1997, 385, 239-241. https://doi.org/10.1038/385239a0.
Steinberg-Yfrach, G.; Rigaud, J.-L.; Durantini, E. N.; Moore, A. L.; Gust, D.; Moore, T. A. Light-driven production of ATP catalysed by F0F1-ATP synthase in an artificial photosynthetic membrane. Nature 1998, 392, 479-482. https://doi.org/10.1038/33116.
Stikane, A.; Hwang, E. T.; Ainsworth, E. V.; Piper, S. E. H.; Critchley, K.; Butt, J. N.; Reisner, E.; Jeuken, L. J. C. Towards compartmentalized photocatalysis: multihaem proteins as transmembrane molecular electron conduits. Faraday Discuss. 2019, 215, 26-38. https://doi.org/10.1039/C8FD00163D.
Su, J.; Chen, H.; Xu, Z.; Wang, S.; Liu, X.; Wang, L.; Huang, X. Near-Infrared-Induced Contractile Proteinosome Microreactor with a Fast Control on Enzymatic Reactions. ACS Applied Materials & Interfaces 2020, 12, 41079-41087. https://doi.org/10.1021/acsami.0c11635.
Sudo, Y.; Toda, F. Photoinduced electron transport across phospholipid wall of liposome using methylene blue. Nature 1979, 279, 807-809. https://doi.org/10.1038/279807a0.
Takizawa, S.-y.; Okuyama, T.; Yamazaki, S.; Sato, K.-i.; Masai, H.; Iwai, T.; Murata, S.; Terao, J. Ion Pairing of Cationic and Anionic Ir(III) Photosensitizers for Photocatalytic CO2 Reduction at Lipid–Membrane Surfaces. J. Am. Chem. Soc. 2023, 145, 15049-15053. https://doi.org/10.1021/jacs.3c03625.
Taku, M.; Kengo, I.; Keisuke, T.; Kunitoshi, H.; Toshihiko, N. A concerted two-step activation of photoinduced electron-transport across lipid membrane. Chem. Lett. 1980, 9, 1009-1012. https://doi.org/10.1246/cl.1980.1009.
Tcherkez, G. G. B.; Farquhar, G. D.; Andrews, T. J. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 7246-7251. https://doi.org/10.1073/pnas.0600605103.
Trantidou, T.; Friddin, M.; Elani, Y.; Brooks, N. J.; Law, R. V.; Seddon, J. M.; Ces, O. Engineering Compartmentalized Biomimetic Micro- and Nanocontainers. ACS Nano 2017, 11, 6549-6565. https://doi.org/10.1021/acsnano.7b03245.
Troppmann, S.; Brandes, E.; Motschmann, H.; Li, F.; Wang, M.; Sun, L.; König, B. Enhanced Photocatalytic Hydrogen Production by Adsorption of an [FeFe]-Hydrogenase Subunit Mimic on Self-Assembled Membranes. Eur. J. Inorg. Chem. 2016, 2016, 554-560. https://doi.org/10.1002/ejic.201501377.
Troppmann, S.; König, B. Functionalized Membranes for Photocatalytic Hydrogen Production. Chem. Eur. J. 2014, 20, 14570-14574. https://doi.org/10.1002/chem.201404480.
Velasco-Garcia, L.; Casadevall, C. Bioinspired photocatalytic systems towards compartmentalized artificial photosynthesis. Commun. Chem. 2023, 6, 263. https://doi.org/10.1038/s42004-023-01069-z.
Walde, P. Building artificial cells and protocell models: Experimental approaches with lipid vesicles. Bioessays 2010, 32, 296-303. https://doi.org/10.1002/bies.200900141.
Walde, P.; Ichikawa, S. Enzymes inside lipid vesicles: preparation, reactivity and applications. Biomol. Eng. 2001, 18, 143-177. https://doi.org/10.1016/S1389-0344(01)00088-0.
Wang, G.; Castiglione, K. Catalysts 2019, 9, 12.
Wang, J.; Zhu, W.; Meng, F.; Bai, G.; Zhang, Q.; Lan, X. Integrating Dual-Metal Sites into Covalent Organic Frameworks for Enhanced Photocatalytic CO2 Reduction. ACS Cat. 2023, 13, 4316-4329. https://doi.org/10.1021/acscatal.3c00126.
Wang, M.; Wölfer, C.; Otrin, L.; Ivanov, I.; Vidaković-Koch, T.; Sundmacher, K. Transmembrane NADH Oxidation with Tetracyanoquinodimethane. Langmuir 2018, 34, 5435-5443. https://doi.org/10.1021/acs.langmuir.8b00443.
Wang, R.; Yuan, Y.; Bang, K.-T.; Kim, Y. Single-Atom Catalysts on Covalent Organic Frameworks for CO2 Reduction. ACS Mater. Au 2023, 3, 28-36. https://doi.org/10.1021/acsmaterialsau.2c00061.
Wang, X.; Yao, C.; Zhang, G.; Liu, S. Regulating vesicle bilayer permeability and selectivity via stimuli-triggered polymersome-to-PICsome transition. Nat. Comm. 2020, 11, 1524. https://doi.org/10.1038/s41467-020-15304-x.
Wegner, T.; Laskar, R.; Glorius, F. Lipid mimetics: A versatile toolbox for lipid biology and beyond. Curr. Opin. Chem. Biol. 2022, 71, 102209. https://doi.org/10.1016/j.cbpa.2022.102209.
Wick, R.; Angelova, M. I.; Walde, P.; Luisi, P. L. Microinjection into giant vesicles and light microscopy investigation of enzyme-mediated vesicle transformations. Chem. Biol. 1996, 3, 105-111. https://doi.org/10.1016/S1074-5521(96)90286-0.
Yang, B.; Li, S.; Mu, W.; Wang, Z.; Han, X. Light-Harvesting Artificial Cells Containing Cyanobacteria for CO2 Fixation and Further Metabolism Mimicking. Small 2023, 19, 2201305. https://doi.org/10.1002/smll.2201305.
Yang, X.-X.; Du, Y.-R.; Li, X.-Q.; Duan, G.-Y.; Chen, Y.-M.; Xu, B.-H. Covalent Organic Frameworks Boost the Silver-Electrocatalyzed Reduction of CO2: The Electronic and Confinement Effect. ACS Appl. Mater. Interfaces 2023, 15, 31533-31542. https://doi.org/10.1021/acsami.3c05679.
Yorulmaz Avsar, S.; Kyropoulou, M.; Di Leone, S.; Schoenenberger, C.; Meier, W. P.; Palivan, C. G. Biomolecules Turn Self-Assembling Amphiphilic Block Co-polymer Platforms Into Biomimetic Interfaces. Front Chem 2018, 6, 645. https://doi.org/10.3389/fchem.2018.00645.
Yoshimoto, M. Stabilization of Enzymes Through Encapsulation in Liposomes. Methods Mol. Biol. 2017, 1504, 9-18. https://doi.org/10.1007/978-1-4939-6499-4_2.
Zhang, B.; Sun, L. Artificial photosynthesis: opportunities and challenges of molecular catalysts. Chem. Soc. Rev. 2019, 48, 2216-2264. https://doi.org/10.1039/C8CS00897C.
Zhang, H.; Casadevall, C.; van Wonderen, J. H.; Su, L.; Butt, J. N.; Reisner, E.; Jeuken, L. J. C. Rational Design of Covalent Multiheme Cytochrome-Carbon Dot Biohybrids for Photoinduced Electron Transfer. Adv. Funct. Mater. 2023, 33, 2302204. https://doi.org/10.1002/adfm.202302204.
Zhang, N.; Trépout, S.; Chen, H.; Li, M.-H. AIE Polymer Micelle/Vesicle Photocatalysts Combined with Native Enzymes for Aerobic Photobiocatalysis J. Am. Chem. Soc. 2023, 145, 288-299. https://doi.org/10.1021/jacs.2c09933.
Zhu, Y.; Cao, S.; Huo, M.; Hest, J. C. M. van; Che, H. Recent advances in permeable polymersomes: fabrication, responsiveness, and applications. Chem. Sci. 2023, 14, 7411- 7437. https://doi.org/10.1039/D3SC01707A.

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
Derechos de autor 2024 Anales de Química de la RSEQ