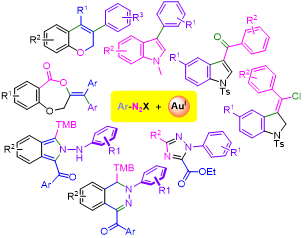
Abstract
In recent years, various protocols that facilitate the oxidation of homogeneous Au(I) catalysts have emerged. The latter has allowed the exploration of the synthetic potential associated with the implementation of Au(I)/Au(III) redox cycles. In this context, our research group has contributed to the development of gold-mediated or -catalysed methodologies that enable the formation of C(S)-aryl bonds, utilizing aryldiazonium salts as electrophiles. This article summarizes the state of the art of research in this area and our contribution to it. Furthermore, it reveals a less-explored type of reactivity in which aryldiazonium salts retain the diazo group, resulting in nitrogen heterocyclic compounds.
References
A. S. K. Hashmi, F. D. Toste (eds.), Modern Gold-Catalyzed Synthesis, Wiley-VCH, Weinheim, 2012, https://doi.org/10.1002/9783527646869.
V. Michelet, F. D. Toste, Gold Catalysis: An Homogeneous Approach, Imperial College Press, London, 2014.
L. Zhang, Acc. Chem. Res. 2014, 47, 877-88, https://doi.org/10.1021/ar400181x.
R. Dorel, A. M. Echavarren, Chem. Rev. 2015, 115, 9028-9072, https://doi.org/10.1021/cr500691k.
R. J. Harris, R. A. Widenhoefer, Chem. Soc. Rev. 2016, 45, 4533-4551, https://doi.org/10.1039/C6CS00171H.
C. C. Chintawar, A. K. Yadav, A. Kumar, S. P. Sancheti, N. T. Patil, Chem. Rev. 2021, 121, 8478-8558; https://doi.org/10.1021/acs.chemrev.0c00903.
T. Wang, A. S. K. Hashmi, Chem. Rev. 2021, 121, 8948-8978, https://doi.org/10.1021/acs.chemrev.0c00811.
G. Zhang, Y. Peng, L. Cui, L. Zhang, Angew. Chem. Int. Ed. 2009, 48, 3112-3115, https://doi.org/10.1002/anie.200900585.
P. Font, H. Valdés, X. Ribas, Angew. Chem. Int. Ed. 2024, 63, e202405824, https://doi.org/10.1002/anie.202405824.
M. Joost, A. Zeineddine, L. Estévez, S. Mallet-Ladeira, K. Miqueu, A. Amgoune, D. Bourissou, J. Am. Chem. Soc. 2014, 136, 14654-14657, https://doi.org/10.1021/ja506978c.
O. Crespo, M. C. Gimeno, A. Laguna, P. G. Jones, J. Chem. Soc. Dalton Trans. 1992, 1601-1605, https://doi.org/10.1039/DT9920001601.
M. C. Gimeno, A. Laguna, Chem. Rev. 1997, 97, 511-522, https://doi.org/10.1021/cr960361q.
O. Crespo, M. C. Gimeno, P. G. Jones, A. Laguna, J. M. López-de-Luzuriaga, M. Monge, J. L. Pérez, M. A. Ramón, Inorg. Chem. 2003, 42, 2061-2038, https://doi.org/10.1021/ic0259843.
R. Visbal, I. Ospino, J. M. López-de-Luzuriaga, A. Laguna, M. C. Gimeno, J. Am. Chem. Soc. 2013, 135, 4712-4715, https://doi.org/10.1021/ja401523x.
I. Fernández, L. P. Wolters, F. M. Bickelhaupt, J. Comput. Chem. 2014, 35, 2140-2145, https://doi.org/10.1002/jcc.23734.
A. Zeineddine, L. Estévez, S. Mallet-Ladeira, K. Miqueu, A. Amgoune, D. Bourissou, Nat. Commun. 2017, 8, 565, https://doi.org/10.1038/s41467-017-00672-8.
M. J. Harper, C. J. Arthur, J. Crosby, E. J. Emmett, R. L. Falconer, A. J. Fensham-Smith, P. J. Gates, T. Leman, J. E. McGrady, J. F. Bower, C. A. Russell, J. Am. Chem. Soc. 2018, 140, 4440-4445, https://doi.org/10.1021/jacs.8b01411.
P. Font, H. Valdés, G. Guisado-Barrios, X. Ribas, Chem. Sci., 2022, 13, 9351-9360, https://doi.org/10.1039/D2SC01966C.
S. C. Scott, J. A. Cadge, G. K. Boden, J. F. Bower, C. A. Russell, Angew. Chem. Int. Ed. 2023, 62, e2023015626, https://doi.org/10.1002/anie.202301526.
P. Gao, J. Xu, T. Zhou, Y. Liu, E. Bisz, B. Dziuk, R. Lalancette, R. Szostak, D. Zhang, M. Szostak, Angew. Chem. Int. Ed. 2023, 62, e202218427, https://doi.org/10.1002/anie.202218427.
K. Muratov, E. Zaripov, M. V. Berezvoski, F. Gagosz, J. Am. Chem. Soc. 2024, 146, 3660-3674, https://doi.org/10.1021/jacs.3c08943.
Urvashi, S. Rai, G. Shukla, Nisha, N. T. Patil, Org. Lett. 2025, 27, 2364-2370, https://doi.org/10.1021/acs.orglett.5c00203.
M. Zhang, C. Zhu, L.-W. Ye, Synthesis 2017, 49, 1150-1157, https://doi.org/10.1055/s-0036-1588365.
A. Nijamudheen, A. Datta, Chem. Eur. J. 2020, 26, 1442-1487, https://doi.org/10.1002/chem.201903377.
I. Medina-Mercado, S. Porcel, Chem. Eur. J. 2020, 26, 16206-16221, https://doi.org/10.1002/chem.202000884.
A. Roglans, A. Pla-Quintana, M. Moreno-Mañas, Chem. Rev. 2006, 106, 4622-4643, https://doi.org/10.1021/cr0509861.
F.-X. Felpin, L. Nassar-Hardy, F. Le Callonnec, E. Fouquet, Tetrahedron 2011, 67, 2815-2831, https://doi.org/10.1016/j.tet.2011.02.051.
F. Mo, G. Dong, Y. Zhang, J. Wang, Org. Biomol. Chem. 2013, 11, 1582-1593, https://doi.org/10.1039/C3OB27366K.
F. Mo, D. Qiui, L. Zhang, J. Wang, Chem. Rev. 2021, 121, 5741-5829, https://doi.org/10.1021/acs.chemrev.0c01030.
J. M. R. Narayanam, C. R. J. Stephenson, Chem. Soc. Rev. 2011, 40, 102-113, https://doi.org/10.1039/B913880N.
C. K. Prier, D. A. Rankic, D. W. C. MacMillan, Chem. Rev. 2013, 113, 5322-5363, https://doi.org/10.1021/cr300503r.
J. C. Tellis, C. B. Kelly, D. N. Primer, M. Jouffroy, N. R. Patel, G. A. Molander, Acc. Chem. Res. 2016, 49, 1429-1439, https://doi.org/10.1021/acs.accounts.6b00214.
A. Y. Chan, I. B. Perry, N. B. Bissonnette, B. F. Buksh, G. A. Edwards, L. I. Frye, O. L. Garry, M. N. Lavagnino, B. X. Li, Y. Liang, E. Mao, A. Millet, J. V. Oakley, N. L. Reed, H. A. Sakai, C. P. Seath, D. W. C. MacMillan, Chem. Rev. 2022, 122, 1485-1542, https://doi.org/10.1021/acs.chemrev.1c00383.
B. Sahoo, M. N. Hopkinson, F. Glorius, J. Am. Chem. Soc. 2013, 135, 5505-5508, https://doi.org/10.1021/ja400311h.
T. Cornilleau, P. Hermange, E. Fouquet, E. Chem. Commun. 2016, 52, 10040-10043, https://doi.org/10.1039/C6CC04239B.
A. Tabey, M. Berlande, P. Hermange, E. Fouquet, Chem. Commun. 2018, 54, 12867-12870, https://doi.org/10.1039/C8CC07530A.
M. Barbero, S. Dughera, S. Tetrahedron, 2018, 74, 5758-5769, https://doi.org/10.1016/j.tet.2018.08.018.
S. Kim, J. Rojas-Martin, F. D. Toste, Chem. Sci. 2016, 7, 85-88, https://doi.org/10.1039/C5SC03025K.
B. Alcaide, P. Almendros, E. Busto, C. Lázaro-Milla, C. J. Org. Chem. 2017, 82, 2177-2186, https://doi.org/10.1021/acs.joc.6b03006.
Alcaide, B.; Almendros, P.; Bustos, E.; Herrera, F.; Lázaro-Milla, C.; Luna, A. Adv. Synth. Catal. 2017, 359, 2640-2652, https://doi.org/10.1002/adsc.201700427.
G. J. Sherborne, A. G. Gevondian, I. Funez-Ardoiz, A. Dahiya, C. Fricke, F. Schoenebeck, Angew. Chem. 2020, 132, 15673; Angew. Chem. Int. Ed. 2020, 59, 15543-15548, https://doi.org/10.1002/anie.202005066.
B. Alcaide, P. Almendros, E. Busto, A. Luna, Adv. Synth. Catal. 2016, 358, 1526-1533, https://doi.org/10.1002/adsc.201600158.
A. Kumar, N. Bhattacharya, N. T. Patil, ChemCatChem 2024, 16, e202401112, https://doi.org/10.1002/cctc.202401112.
S. Zhang, X. Ye, L. Wojtas, W. Hao, X. Shi, Green Synth. Catal. 2021, 2, 82-86, https://doi.org/10.1016/j.gresc.2021.01.008.
H. Liang, Y. Julaiti, C.-G. Zhao, J. Xie, Nat. Synth. 2023, 2, 338-347, https://doi.org/10.1038/s44160-022-00219-w.
L. Huang, M. Rudolph, F. Rominger, A. S. K. Hashmi, J. Xie, Angew. Chem. Int. Ed. 2016, 55, 4808-4813, https://doi.org/10.1002/anie.201511487.
R. Cai, M. Lu, E. Y. Aguilera, Y. Xi, N. G. Akhmedov, J. L. Peterson, H. Chen, X. Shi, Angew. Chem. Int. Ed. 2015, 54, 8772-8776, https://doi.org/10.1002/anie.201503546.
E. O. Asomoza-Solís, J. Rojas-Ocampo, R. A. Toscano, S. Porcel, 2016, 52, 7295-7298, https://doi.org/10.1039/C6CC03105F.
U. A. Carrillo-Arcos, S. Porcel, Org. Biomol. Chem. 2018, 16, 1837-1842, https://doi.org/10.1039/C7OB02447A.
U. Costas-Costas, E. González-Romero, C. Bravo-Díaz, Helv. Chim. Acta 2001, 84, 632-648, https://doi.org/10.1002/1522-2675(20010321)84:3%3C632::AID-HLCA632%3E3.0.CO;2-0
F. P. Crisóstomo, T. Martín, R. Carrillo, Angew. Chem. Int. Ed. 2014, 53, 2181-2185, https://doi.org/10.1002/anie.201309761.
I. Medina-Mercado, E. O. Asomoza-Solís, E. Martínez-González, V. M. Ugalde-Saldívar, L. G. Ledesma-Olvera, J. E. Barquera-Lozada, V. Gómez-Vidales, J. Barroso-Flores, B. A. Frontana-Uribe, S. Porcel, Chem. Eur. J. 2020, 26, 634-642, https://doi.org/10.1002/chem.201904413.
I. Medina-Mercado, A. Colin-Molina, J. E. Barquera-Lozada, B. Rodríguez-Molina, S. Porcel, ACS Catal. 2021, 11, 8968-8977, https://doi.org/10.1021/acscatal.1c01826.
S. Darses, T. Jeffery, J.-P. Gênet, J.-L. Brayer, J.-P. Demoute, Tetrahedron Lett. 1996, 37, 3857-3860, https://doi.org/10.1016/0040-4039(96)00699-5.
F.-X. Felpin, S. Sengupta, Chem. Soc. Rev. 2019, 48, 1150-1193, https://doi.org/10.1039/C8CS00453F.
H. Bonin, E. Fouquet, F.-X. Felpin, Adv. Synth. Catal. 2011, 353, 3063-3084, https://doi.org/10.1002/adsc.201100531.
I. Medina-Mercado, S. Porcel, Synthesis 2022, 54, 5077-5088, https://doi.org/10.1055/s-0041-1737882.
O. Stadler, Ber. Dtsch. Chem. Ges. 1884, 17, 2075-2081, https://doi.org/10.1002/cber.188401702106.
L. Currie, L. Rocchigiani, D. L. Hughes, M. Bochmann, Dalton Trans. 2018, 47, 6333–6343, https://doi.org/10.1039/C8DT00906F.
M. S. Messina, J. M. Stauber, M. A. Waddington, A. L. Rheingold, H. D. Maynard, A. M. Spokoyny, J. Am. Chem. Soc. 2018, 140, 7065–7069, https://doi.org/10.1021/jacs.8b04115.
M. N. Wenzel, R. Bonsignore, S. R. Thomas, D. Bourissou, G. Barone, A. Casini, Chem. Eur. J. 2019, 25, 7628-764, https://doi.org/10.1002/chem.201901535.
S.-L. Zhang, J.-J. Dong, Org. Biomol. Chem. 2019, 17, 1245-1253, https://doi.org/10.1039/C8OB03143F.
A. Caballero-Muñoz, M. Rosas-Ortega, H. Díaz-Salazar, S. Porcel, Eur. J. Org. Chem. 2023, 26 e202300203, https://doi.org/10.1002/ejoc.202300203.
M. Pernpointner, A. S. K. Hashmi, J. Chem. Theory Comput. 2009, 5, 2717-2725, https://doi.org/10.1021/ct900441f.
L. Gregori, D. Sorbelli, L. Belpassi, F. Tarantelli, P. Belanzoni, Inorg. Chem. 2019, 58, 3115-3129, https://doi.org/10.1021/acs.inorgchem.8b03172.
J.-R. Deng, W.-C. Chang, N. C.-H. Lai, B. Yang, C.-S. Tsang, B. C.-B. Ko, S. L-F. Chan, M.-K. Wong, Chem. Sci. 2017, 8, 7537-7544, https://doi.org/10.1039/C7SC02294H.
L. Rocchigiani, J. Fernandez-Cestau, G. Agonigi, I. Chambrier, P. H. M. Budzelaar, M. Bochmann, Angew. Chem. Int. Ed. 2017, 56, 13861-13865, https://doi.org/10.1002/anie.201708640.
H. Díaz-Salazar, I. Medina-Mercado, R. Salvador-Reyes, J. E. Barquera-Lozada, D. Martínez-Otero, S. Porcel, Chem. Eur. J. 2023, 29, e202302074, https://doi.org/10.1002/chem.202302074.
H. Schmidbaur, H. G. Raubenheimer, L. Dobrzanska, Chem. Soc. Rev. 2014, 43, 345-380, https://doi.org/10.1039/C3CS60251F.
S. Taschinski, R. Döpp, M. Ackermann, F. Rominger, F. De Vries, M. F. S. J. Menger, M. Rudolph, A. S. K. Hashmi, J. E. M. N. Klein, Angew. Chem. Int. Ed. 2019, 58, 16988-16993, https://doi.org/10.1002/anie.201908268.
T. Yuan, Q. Tang, C. Shan, X. Ye, J. Wang, P. Zhao, L. Wotjas, N. Hadler, H. Chen, X. Shi, J. Am. Chem. Soc. 2021, 143, 4074-4082, https://doi.org/10.1021/jacs.1c01811.
J. Li, H. Shi, S. Zhang, M. Rudolph, F. Rominger, A. S. K. Hashmi, Org. Lett. 2021, 23, 7713-7717, https://doi.org/10.1021/acs.orglett.1c02621.
H. Díaz-Salazar, G. Osorio-Ocampo, S. Porcel, J. Org. Chem. 2024, 89, 10163-10174, https://doi.org/10.1021/acs.joc.4c01039.
H. Díaz-Salazar, C. M. Ramírez-González, M. A. Rosas-Ortega, S. Porcel, Tetrahedron 2024, 168, 134358, https://doi.org/10.1016/j.tet.2024.134358
La formación del iluro de nitrilo y la cicloadición [3+2] han sido estudiadas por cálculos teóricos, en reacciones similares catalizadas por Cu(I): H. Li, X. Wu, W. Hao, H. Li, Y. Zhao, Y. Wang, P. Lian, Y. Zheng, X. Bao, X. Wan, Org. Lett. 2018, 20, 5224-5227, https://doi.org/10.1021/acs.orglett.8b02172.
E. R. M. Habraken, A. R. Jupp, J. C. Slootweg, Synlett 2019, 30, 875-884, https://doi.org/10.1055/s-0037-1612109.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Copyright (c) 2025 Anales de Química de la RSEQ