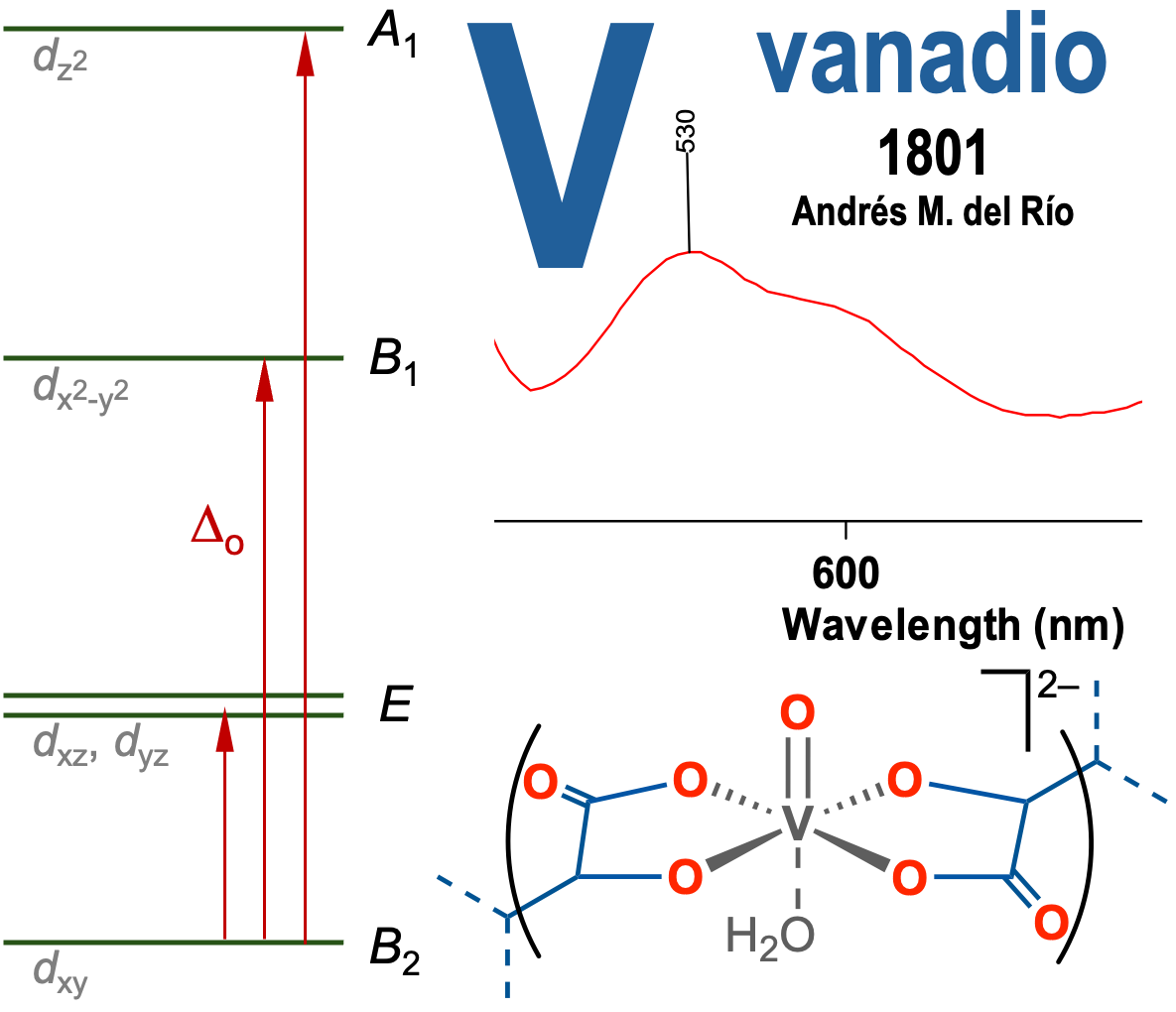
Abstract
Vanadium, the element discovered by Andrés Manuel del Río, has an aqueous chemistry very suitable for
laboratory experiments, in which the availability of different contiguous and accessible oxidation states, the
acid-base behavior of its compounds or the coordination chemistry and spectroscopic properties of its complexes
can be exploited. In addition to its didactic value, it is also attractive due to the beautiful color of its
combinations. This contribution describes activities involving vanadium compounds that have been carried
out in advanced inorganic chemistry courses at our University for years. The main objective is to have students
record and analyze the electronic spectra of species of the metal with different d-configurations.
References
F. Carrillo Hermosilla, An. Quím. 2019, 115, 85.
P. Román Polo, An. Quím. 2019, 115, 136.
G. Pinto Cañón, An. Quím. 2019, 115, 140.
L. Romero, “El eritronio, antecedente del vanadio: Aportación hispano-mexicana a la Tabla Periódica de elementos”, Gaceta UNAM, disponible en https://bit.ly/3H3Razt, Feb 18, 2019 (consultado: 01/07/2025).
Royal Society of Chemistry, “The oxidation states of vanadium”, disponible en https://bit.ly/3MDEmPN, (consultado: 01/07/2025).
W.D. Bare, W. Resto, J. Chem. Educ. 1994, 71, 692-694, https://doi.org/10.1021/ed071p692.
LibreTexts, “Vanadium rainbow”, disponible en https://bit.ly/3Tmftfn, (consultado: 01/07/2025).
J. Clark, S.R. Marsden “Chemistry of vanadium”, disponible en https://bit.ly/3MFEePO, (consultado: 01/07/2025).
R.A.D. Wentworth, J. Chem. Educ. 1985, 62, 440-441, https://doi.org/10.1021/ed062p440.
A. Ward Grant Jr., J. Chem. Educ. 1977, 54, 500, https://doi.org/10.1021/ed054p500.
J.M. Davis, J. Chem. Educ. 1968, 45, 473, https://doi.org/10.1021/ed045p473.
F.C. Hentz Jr., G.G. Long, J. Chem. Educ. 1978, 55, 55-56, https://doi.org/10.1021/ed055p55.
W. G. Palmer, Experimental Inorganic Chemistry, Cambrige University Press, 1970, 276-278 y 318-322.
Z. Szafran, R.M. Pike, M.M. Singh, Microscale Inorganic Chemistry: A Comprehensive Laboratory Experience, John Wiley & Sons, 1991, 246-248.
C. Glidewell, en Inorganic Experiments (Ed.: J.D. Woollins), John Wiley & Sons, 2010, 109-111.
C.P. Morley en Inorganic Experiments (Ed.: J. D. Woollins), John Wiley & Sons, 2010, 142-144.
C.E. Ophardt, S. Stupgia, J. Chem. Educ. 1984, 61, 1102-1103, https://doi.org/10.1021/ed061p1102.
S.M. Malinak, J.E. Hertzog, J.E. Pacilio, D.A. Polvani, J. Chem. Educ. 2019, 96, 582-585, https://doi.org/10.1021/acs.jchemed.8b00543.
K.D.M. Charleton, E.M. Prokopchuk, J. Chem. Educ. 2011, 88, 1155-1157, https://doi.org/10.1021/ed100843a.
K. Elen, A. Hardy, M.K. Van Bael, J. Chem. Educ. 2020, 97, 1650-1654, https://doi.org/10.1021/acs.jchemed.0c00010.
C.E. Housecroft, A.G. Sharpe, Inorganic Chemistry, Pearson, 2018.
J.J. Cruywagen, J.B.B. Heyns, E.A. Rohwer, J. Chem. Soc. Dalton Trans. 1990, 1951-1956, https://doi.org/10.1039/DT9900001951.
M. Seco, J. Chem. Educ. 1989, 66, 779-780, https://doi.org/10.1021/ed066p779.
G.A. Bain, J.F. Berry, J. Chem. Educ. 2008, 85, 532-536, https://doi.org/10.1021/ed085p532.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Copyright (c) 2025 Anales de Química de la RSEQ