Abstract
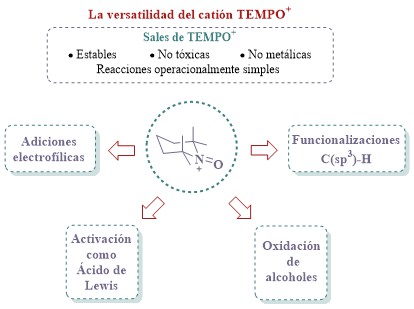
Cation derived from the tetramethylpiperidine-N-oxoammonium (TEMPO+) have found several applications in organic synthesis, mainly as oxidizing agents for obtain-ing carbonyls from alcohols. TEMPO+ has also proven to be highly efficient and selective for promoting C(sp3)–H functionalization of N-heterocycles. Recently, it was dis- covered that can act as Lewis acid in electrophilic addition reactions, Nazarov and Ferrier rearrangement. Owing to their non-metallic nature, high stability, and low toxicity, TEMPO+ salts are highly valuable and affordable reagents. In this article, we will briefly explore the evolution of the chemistry of the TEMPO+ and its application in different chemical transformations. We will show some representative examples and describe certain key reaction mechanisms to highlight the privileged role that TEMPO+ plays in organic synthesis.
References
V. A. Golubev, É. G. Rozantsev, M. B. Neiman, Izv. Akad. Nauk SSSR, Ser. Khim. 1965, 11, 1927 (Bull. Acad. Sci. USSR, Chem. Sci. 1965, 14,1898–1904). https://doi.org/10.1007/BF00845878.
A. E. J. de Nooy, A. C. Besemer, H. van Bekkum, Synthesis, 1996, 10, 1153-1176. https://doi.org/10.1055/s-1996-4369.
J. M. Bobbitt, C. Brückner, N. Merbouh, Org. React. 2009, 74, 103–424. https://doi.org/10.1002/0471264180.or074.02.
Nitroxide‐catalyzed alcohol oxidations in organic synthesis. Stable Radicals. C. Brückner, Wiley, July 16, 2010, pp 433–460. https://doi.org/10.1002/9780470666975.ch12.
S. Cruz-Gregorio, J. Romero-Ibañez, F. Sartillo-Piscil, Targets Heterocycl. Syst. 2022, 26, 100–121. https://doi.org/10.17374/targets.2023.26.100
L. Porras-Santos, J. Sandoval-Lira, L. Quintero, J. M. Hernández-Pérez, P. López-Mendoza, F. Sartillo-Piscil, J. Org. Chem. 2024, 89, 11281–11292. https://doi.org/10.1021/acs.joc.4c00978.
J. Bautista-Nava, L. Porras-Santos, A. Pérez-Bautista, L. Quintero, P. López-Mendoza, F. Sartillo-Piscil, J. Org. Chem. 2025, 90, 6251–6260. https://doi.org/10.1021/acs.joc.5c00354.
V. A. Golubev, V. N. Borislavskii, A. L. Aleksandrov, Russ. Chem. Bull. 1977, 9, 1874–1881. (Traducido al Inglés). https://doi.org/10.1007/BF00924380.
M.F. Semmelhack, C.R. Schmid, D.A Cortés, Tetrahedron Lett. 1986, 27, 1119–1122. https://doi.org/10.1016/S0040-4039(00)84193-3.
W. F. Bailey, J. M. Bobbitt, K.B. Wiberg, J. Org. Chem. 2007, 72, 4504−4509. https://doi.org/10.1021/jo0704614.
J. M. Bobbitt, A. L. Bartelson, W. F. Bailey, T. A. Hamlin, C. B. Kelly, C. B., J. Org. Chem. 2014, 79, 1055–1067. https://doi.org/10.1021/jo402519m.
T. A Hamlin, C. B. Kelly, J. M. Ovian, R. J. Wiles, L. J. Tilley, N. E. Leadbeater, J. Org. Chem. 2015, 80, 8150–8167. https://doi.org/10.1021/acs.joc.5b01240.
H. Richter, O. García-Mancheño, Eur. J. Org. Chem. 2010, 23, 4460–4467. https://doi.org/10.1002/ejoc.201000548.
Y. Changcun, L. Yuxiu, W. Qingmin, Org. Lett. 2015, 17, 5714–5717. https://doi.org/10.1021/acs.orglett.5b03042.
U. Osorio-Nieto, D. Chamorro-Arenas, L. Quintero, H. Höpfl, F. Sartillo-Piscil, J. Org. Chem. 2016, 81, 8625−8632. https://doi.org/10.1021/acs.joc.6b01566.
J. Romero-Ibañez, S. Cruz-Gregorio, J. Sandoval-Lira, J. M. Hernández-Párez, L. Quintero, F. Sartillo-Piscil, F. Angew.Chem. Int. Ed. 2019, 58, 8867−8871. https://doi.org/10.1002/anie.201903880.
A. Recoba-Torres, S. Cruz-Gregorio, L. Quintero, J. Sandoval-Lira, J. Romero-Ibañez, F. Sartillo-Piscil. Eur. J. Org. Chem. 2022, e202200292. https://doi.org/10.1002/ejoc.202200292.
Á. A. Nolasco-Hernández, L. Quintero, S. Cruz-Gregorio, F. Sartillo-Piscil, J. Org. Chem. 2024, 89, 1762–1768. https://doi.org/10.1021/acs.joc.3c02466.
J. Rivera-Mendoza, Á. A. Nolasco-Hernández, L. Quintero, V. Carranza-Téllez, F. Sartillo-Piscil, Tetrahedron 2025, 185, 134800. https://doi.org/10.1016/j.tet.2025.134800.
Y. He, Q. Liu, T. Gong, Y. Liu, X. Zhang, X. Fan, Org. Chem. Front. 2023, 10, 1206–1212. https://doi.org/10.1039/D2QO01427K.
S. Masatoshi, T. Masaki, Y. Iwabuchi, J. Org. Chem. 2008, 73, 4750–4752, https://doi.org/10.1021/jo800634r.
Y. Zhang, Y. Chen, M. Song, B. Tan, Y. Jiang, C. Yan, Y. Jiang, X. Hu, C. Zhang, W. Chen, J. Xu, J. Am. Chem. Soc. 2022, 144, 16042−16051. https://doi.org/10.1021/jacs.2c05957.
K. Moriyama, M. Kuramochi, S.Tsuzuki, K. Fujii, T. Morita, Org. Lett. 2021, 23, 268−273. https://doi.org/10.1021/acs.orglett.0c03546.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Copyright (c) 2025 Anales de Química de la RSEQ