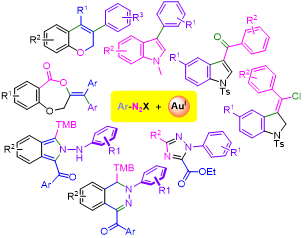
Resumen
En los últimos años han emergido varias estrategias que facilitan la oxidación de catalizadores homogéneos de Au(I), permitiendo la exploración del potencial sintético asociado a ciclos rédox Au(I)/Au(III). En este contexto, nuestro grupo de investigación ha participado en el desarrollo de metodologías que posibilitan la creación de enlaces C(S)-arilo, empleando sales de arildiazonio como electrófilos. El presente artículo resume el estado del arte de la investigación en esta área y nuestra contribución al respecto. Además, muestra un tipo de reactividad menos explorada, en la que las sales de arildiazonio conservan el grupo diazo, lo que conduce a la formación de heterociclos nitrogenados.
Referencias
A. S. K. Hashmi, F. D. Toste (eds.), Modern Gold-Catalyzed Synthesis, Wiley-VCH, Weinheim, 2012, https://doi.org/10.1002/9783527646869.
V. Michelet, F. D. Toste, Gold Catalysis: An Homogeneous Approach, Imperial College Press, London, 2014.
L. Zhang, Acc. Chem. Res. 2014, 47, 877-88, https://doi.org/10.1021/ar400181x.
R. Dorel, A. M. Echavarren, Chem. Rev. 2015, 115, 9028-9072, https://doi.org/10.1021/cr500691k.
R. J. Harris, R. A. Widenhoefer, Chem. Soc. Rev. 2016, 45, 4533-4551, https://doi.org/10.1039/C6CS00171H.
C. C. Chintawar, A. K. Yadav, A. Kumar, S. P. Sancheti, N. T. Patil, Chem. Rev. 2021, 121, 8478-8558; https://doi.org/10.1021/acs.chemrev.0c00903.
T. Wang, A. S. K. Hashmi, Chem. Rev. 2021, 121, 8948-8978, https://doi.org/10.1021/acs.chemrev.0c00811.
G. Zhang, Y. Peng, L. Cui, L. Zhang, Angew. Chem. Int. Ed. 2009, 48, 3112-3115, https://doi.org/10.1002/anie.200900585.
P. Font, H. Valdés, X. Ribas, Angew. Chem. Int. Ed. 2024, 63, e202405824, https://doi.org/10.1002/anie.202405824.
M. Joost, A. Zeineddine, L. Estévez, S. Mallet-Ladeira, K. Miqueu, A. Amgoune, D. Bourissou, J. Am. Chem. Soc. 2014, 136, 14654-14657, https://doi.org/10.1021/ja506978c.
O. Crespo, M. C. Gimeno, A. Laguna, P. G. Jones, J. Chem. Soc. Dalton Trans. 1992, 1601-1605, https://doi.org/10.1039/DT9920001601.
M. C. Gimeno, A. Laguna, Chem. Rev. 1997, 97, 511-522, https://doi.org/10.1021/cr960361q.
O. Crespo, M. C. Gimeno, P. G. Jones, A. Laguna, J. M. López-de-Luzuriaga, M. Monge, J. L. Pérez, M. A. Ramón, Inorg. Chem. 2003, 42, 2061-2038, https://doi.org/10.1021/ic0259843.
R. Visbal, I. Ospino, J. M. López-de-Luzuriaga, A. Laguna, M. C. Gimeno, J. Am. Chem. Soc. 2013, 135, 4712-4715, https://doi.org/10.1021/ja401523x.
I. Fernández, L. P. Wolters, F. M. Bickelhaupt, J. Comput. Chem. 2014, 35, 2140-2145, https://doi.org/10.1002/jcc.23734.
A. Zeineddine, L. Estévez, S. Mallet-Ladeira, K. Miqueu, A. Amgoune, D. Bourissou, Nat. Commun. 2017, 8, 565, https://doi.org/10.1038/s41467-017-00672-8.
M. J. Harper, C. J. Arthur, J. Crosby, E. J. Emmett, R. L. Falconer, A. J. Fensham-Smith, P. J. Gates, T. Leman, J. E. McGrady, J. F. Bower, C. A. Russell, J. Am. Chem. Soc. 2018, 140, 4440-4445, https://doi.org/10.1021/jacs.8b01411.
P. Font, H. Valdés, G. Guisado-Barrios, X. Ribas, Chem. Sci., 2022, 13, 9351-9360, https://doi.org/10.1039/D2SC01966C.
S. C. Scott, J. A. Cadge, G. K. Boden, J. F. Bower, C. A. Russell, Angew. Chem. Int. Ed. 2023, 62, e2023015626, https://doi.org/10.1002/anie.202301526.
P. Gao, J. Xu, T. Zhou, Y. Liu, E. Bisz, B. Dziuk, R. Lalancette, R. Szostak, D. Zhang, M. Szostak, Angew. Chem. Int. Ed. 2023, 62, e202218427, https://doi.org/10.1002/anie.202218427.
K. Muratov, E. Zaripov, M. V. Berezvoski, F. Gagosz, J. Am. Chem. Soc. 2024, 146, 3660-3674, https://doi.org/10.1021/jacs.3c08943.
Urvashi, S. Rai, G. Shukla, Nisha, N. T. Patil, Org. Lett. 2025, 27, 2364-2370, https://doi.org/10.1021/acs.orglett.5c00203.
M. Zhang, C. Zhu, L.-W. Ye, Synthesis 2017, 49, 1150-1157, https://doi.org/10.1055/s-0036-1588365.
A. Nijamudheen, A. Datta, Chem. Eur. J. 2020, 26, 1442-1487, https://doi.org/10.1002/chem.201903377.
I. Medina-Mercado, S. Porcel, Chem. Eur. J. 2020, 26, 16206-16221, https://doi.org/10.1002/chem.202000884.
A. Roglans, A. Pla-Quintana, M. Moreno-Mañas, Chem. Rev. 2006, 106, 4622-4643, https://doi.org/10.1021/cr0509861.
F.-X. Felpin, L. Nassar-Hardy, F. Le Callonnec, E. Fouquet, Tetrahedron 2011, 67, 2815-2831, https://doi.org/10.1016/j.tet.2011.02.051.
F. Mo, G. Dong, Y. Zhang, J. Wang, Org. Biomol. Chem. 2013, 11, 1582-1593, https://doi.org/10.1039/C3OB27366K.
F. Mo, D. Qiui, L. Zhang, J. Wang, Chem. Rev. 2021, 121, 5741-5829, https://doi.org/10.1021/acs.chemrev.0c01030.
J. M. R. Narayanam, C. R. J. Stephenson, Chem. Soc. Rev. 2011, 40, 102-113, https://doi.org/10.1039/B913880N.
C. K. Prier, D. A. Rankic, D. W. C. MacMillan, Chem. Rev. 2013, 113, 5322-5363, https://doi.org/10.1021/cr300503r.
J. C. Tellis, C. B. Kelly, D. N. Primer, M. Jouffroy, N. R. Patel, G. A. Molander, Acc. Chem. Res. 2016, 49, 1429-1439, https://doi.org/10.1021/acs.accounts.6b00214.
A. Y. Chan, I. B. Perry, N. B. Bissonnette, B. F. Buksh, G. A. Edwards, L. I. Frye, O. L. Garry, M. N. Lavagnino, B. X. Li, Y. Liang, E. Mao, A. Millet, J. V. Oakley, N. L. Reed, H. A. Sakai, C. P. Seath, D. W. C. MacMillan, Chem. Rev. 2022, 122, 1485-1542, https://doi.org/10.1021/acs.chemrev.1c00383.
B. Sahoo, M. N. Hopkinson, F. Glorius, J. Am. Chem. Soc. 2013, 135, 5505-5508, https://doi.org/10.1021/ja400311h.
T. Cornilleau, P. Hermange, E. Fouquet, E. Chem. Commun. 2016, 52, 10040-10043, https://doi.org/10.1039/C6CC04239B.
A. Tabey, M. Berlande, P. Hermange, E. Fouquet, Chem. Commun. 2018, 54, 12867-12870, https://doi.org/10.1039/C8CC07530A.
M. Barbero, S. Dughera, S. Tetrahedron, 2018, 74, 5758-5769, https://doi.org/10.1016/j.tet.2018.08.018.
S. Kim, J. Rojas-Martin, F. D. Toste, Chem. Sci. 2016, 7, 85-88, https://doi.org/10.1039/C5SC03025K.
B. Alcaide, P. Almendros, E. Busto, C. Lázaro-Milla, C. J. Org. Chem. 2017, 82, 2177-2186, https://doi.org/10.1021/acs.joc.6b03006.
Alcaide, B.; Almendros, P.; Bustos, E.; Herrera, F.; Lázaro-Milla, C.; Luna, A. Adv. Synth. Catal. 2017, 359, 2640-2652, https://doi.org/10.1002/adsc.201700427.
G. J. Sherborne, A. G. Gevondian, I. Funez-Ardoiz, A. Dahiya, C. Fricke, F. Schoenebeck, Angew. Chem. 2020, 132, 15673; Angew. Chem. Int. Ed. 2020, 59, 15543-15548, https://doi.org/10.1002/anie.202005066.
B. Alcaide, P. Almendros, E. Busto, A. Luna, Adv. Synth. Catal. 2016, 358, 1526-1533, https://doi.org/10.1002/adsc.201600158.
A. Kumar, N. Bhattacharya, N. T. Patil, ChemCatChem 2024, 16, e202401112, https://doi.org/10.1002/cctc.202401112.
S. Zhang, X. Ye, L. Wojtas, W. Hao, X. Shi, Green Synth. Catal. 2021, 2, 82-86, https://doi.org/10.1016/j.gresc.2021.01.008.
H. Liang, Y. Julaiti, C.-G. Zhao, J. Xie, Nat. Synth. 2023, 2, 338-347, https://doi.org/10.1038/s44160-022-00219-w.
L. Huang, M. Rudolph, F. Rominger, A. S. K. Hashmi, J. Xie, Angew. Chem. Int. Ed. 2016, 55, 4808-4813, https://doi.org/10.1002/anie.201511487.
R. Cai, M. Lu, E. Y. Aguilera, Y. Xi, N. G. Akhmedov, J. L. Peterson, H. Chen, X. Shi, Angew. Chem. Int. Ed. 2015, 54, 8772-8776, https://doi.org/10.1002/anie.201503546.
E. O. Asomoza-Solís, J. Rojas-Ocampo, R. A. Toscano, S. Porcel, 2016, 52, 7295-7298, https://doi.org/10.1039/C6CC03105F.
U. A. Carrillo-Arcos, S. Porcel, Org. Biomol. Chem. 2018, 16, 1837-1842, https://doi.org/10.1039/C7OB02447A.
U. Costas-Costas, E. González-Romero, C. Bravo-Díaz, Helv. Chim. Acta 2001, 84, 632-648, https://doi.org/10.1002/1522-2675(20010321)84:3%3C632::AID-HLCA632%3E3.0.CO;2-0
F. P. Crisóstomo, T. Martín, R. Carrillo, Angew. Chem. Int. Ed. 2014, 53, 2181-2185, https://doi.org/10.1002/anie.201309761.
I. Medina-Mercado, E. O. Asomoza-Solís, E. Martínez-González, V. M. Ugalde-Saldívar, L. G. Ledesma-Olvera, J. E. Barquera-Lozada, V. Gómez-Vidales, J. Barroso-Flores, B. A. Frontana-Uribe, S. Porcel, Chem. Eur. J. 2020, 26, 634-642, https://doi.org/10.1002/chem.201904413.
I. Medina-Mercado, A. Colin-Molina, J. E. Barquera-Lozada, B. Rodríguez-Molina, S. Porcel, ACS Catal. 2021, 11, 8968-8977, https://doi.org/10.1021/acscatal.1c01826.
S. Darses, T. Jeffery, J.-P. Gênet, J.-L. Brayer, J.-P. Demoute, Tetrahedron Lett. 1996, 37, 3857-3860, https://doi.org/10.1016/0040-4039(96)00699-5.
F.-X. Felpin, S. Sengupta, Chem. Soc. Rev. 2019, 48, 1150-1193, https://doi.org/10.1039/C8CS00453F.
H. Bonin, E. Fouquet, F.-X. Felpin, Adv. Synth. Catal. 2011, 353, 3063-3084, https://doi.org/10.1002/adsc.201100531.
I. Medina-Mercado, S. Porcel, Synthesis 2022, 54, 5077-5088, https://doi.org/10.1055/s-0041-1737882.
O. Stadler, Ber. Dtsch. Chem. Ges. 1884, 17, 2075-2081, https://doi.org/10.1002/cber.188401702106.
L. Currie, L. Rocchigiani, D. L. Hughes, M. Bochmann, Dalton Trans. 2018, 47, 6333–6343, https://doi.org/10.1039/C8DT00906F.
M. S. Messina, J. M. Stauber, M. A. Waddington, A. L. Rheingold, H. D. Maynard, A. M. Spokoyny, J. Am. Chem. Soc. 2018, 140, 7065–7069, https://doi.org/10.1021/jacs.8b04115.
M. N. Wenzel, R. Bonsignore, S. R. Thomas, D. Bourissou, G. Barone, A. Casini, Chem. Eur. J. 2019, 25, 7628-764, https://doi.org/10.1002/chem.201901535.
S.-L. Zhang, J.-J. Dong, Org. Biomol. Chem. 2019, 17, 1245-1253, https://doi.org/10.1039/C8OB03143F.
A. Caballero-Muñoz, M. Rosas-Ortega, H. Díaz-Salazar, S. Porcel, Eur. J. Org. Chem. 2023, 26 e202300203, https://doi.org/10.1002/ejoc.202300203.
M. Pernpointner, A. S. K. Hashmi, J. Chem. Theory Comput. 2009, 5, 2717-2725, https://doi.org/10.1021/ct900441f.
L. Gregori, D. Sorbelli, L. Belpassi, F. Tarantelli, P. Belanzoni, Inorg. Chem. 2019, 58, 3115-3129, https://doi.org/10.1021/acs.inorgchem.8b03172.
J.-R. Deng, W.-C. Chang, N. C.-H. Lai, B. Yang, C.-S. Tsang, B. C.-B. Ko, S. L-F. Chan, M.-K. Wong, Chem. Sci. 2017, 8, 7537-7544, https://doi.org/10.1039/C7SC02294H.
L. Rocchigiani, J. Fernandez-Cestau, G. Agonigi, I. Chambrier, P. H. M. Budzelaar, M. Bochmann, Angew. Chem. Int. Ed. 2017, 56, 13861-13865, https://doi.org/10.1002/anie.201708640.
H. Díaz-Salazar, I. Medina-Mercado, R. Salvador-Reyes, J. E. Barquera-Lozada, D. Martínez-Otero, S. Porcel, Chem. Eur. J. 2023, 29, e202302074, https://doi.org/10.1002/chem.202302074.
H. Schmidbaur, H. G. Raubenheimer, L. Dobrzanska, Chem. Soc. Rev. 2014, 43, 345-380, https://doi.org/10.1039/C3CS60251F.
S. Taschinski, R. Döpp, M. Ackermann, F. Rominger, F. De Vries, M. F. S. J. Menger, M. Rudolph, A. S. K. Hashmi, J. E. M. N. Klein, Angew. Chem. Int. Ed. 2019, 58, 16988-16993, https://doi.org/10.1002/anie.201908268.
T. Yuan, Q. Tang, C. Shan, X. Ye, J. Wang, P. Zhao, L. Wotjas, N. Hadler, H. Chen, X. Shi, J. Am. Chem. Soc. 2021, 143, 4074-4082, https://doi.org/10.1021/jacs.1c01811.
J. Li, H. Shi, S. Zhang, M. Rudolph, F. Rominger, A. S. K. Hashmi, Org. Lett. 2021, 23, 7713-7717, https://doi.org/10.1021/acs.orglett.1c02621.
H. Díaz-Salazar, G. Osorio-Ocampo, S. Porcel, J. Org. Chem. 2024, 89, 10163-10174, https://doi.org/10.1021/acs.joc.4c01039.
H. Díaz-Salazar, C. M. Ramírez-González, M. A. Rosas-Ortega, S. Porcel, Tetrahedron 2024, 168, 134358, https://doi.org/10.1016/j.tet.2024.134358
La formación del iluro de nitrilo y la cicloadición [3+2] han sido estudiadas por cálculos teóricos, en reacciones similares catalizadas por Cu(I): H. Li, X. Wu, W. Hao, H. Li, Y. Zhao, Y. Wang, P. Lian, Y. Zheng, X. Bao, X. Wan, Org. Lett. 2018, 20, 5224-5227, https://doi.org/10.1021/acs.orglett.8b02172.
E. R. M. Habraken, A. R. Jupp, J. C. Slootweg, Synlett 2019, 30, 875-884, https://doi.org/10.1055/s-0037-1612109.

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
Derechos de autor 2025 Anales de Química de la RSEQ