Abstract
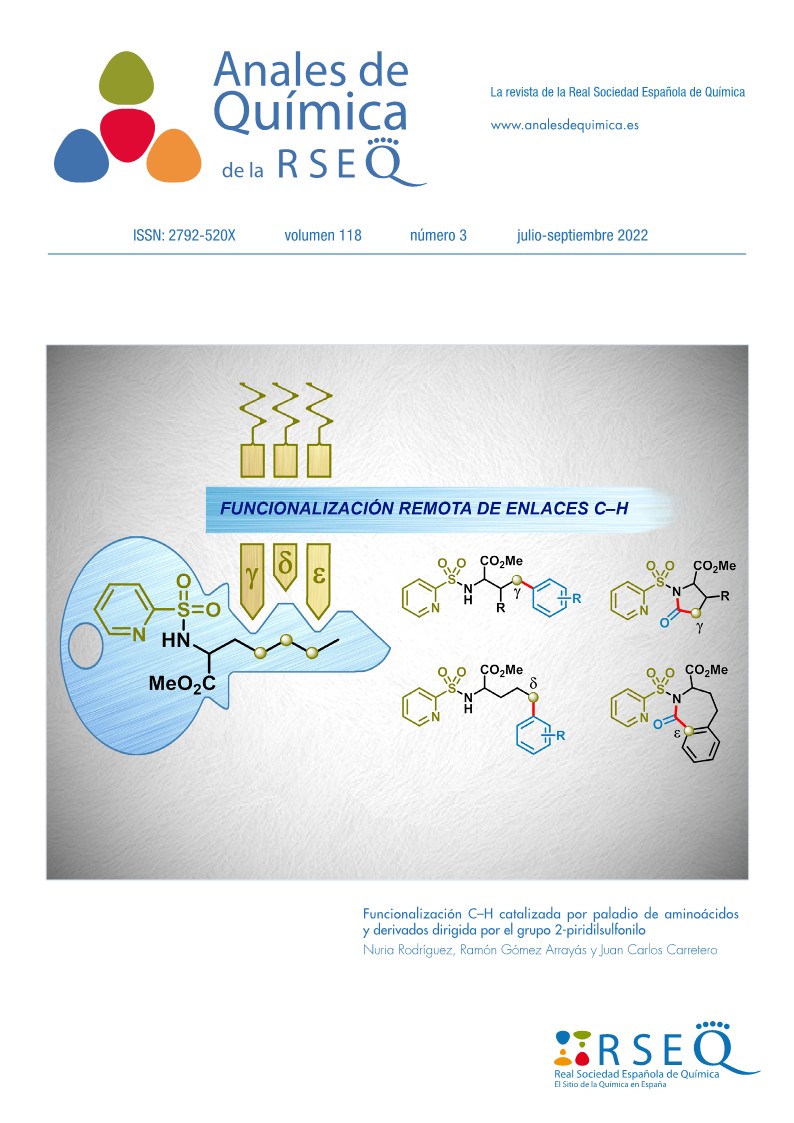
The amino acids are of crucial importance in many research areas. This article describes a variety of C–H functionalization reactions of amino acids catalyzed by Pd in diverse positions of the carbon chain. In particular, we have developed robust and selective procedures for the γ- and δ-arylation, as well as γ- and ε-carbonylation with formation of γ-lactames and benzazepinones, respectively. These methodologies have been also extended to small peptides. In all these C–H functionalization reactions the coordinating group SO2Py plays a crucial role and can be eliminated in the final products under mild reductive conditions.

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Copyright (c) 2022 Nuria Rodríguez , Ramón Gómez Arrayás, Juan Carlos Carretero